Looking for Bacteria
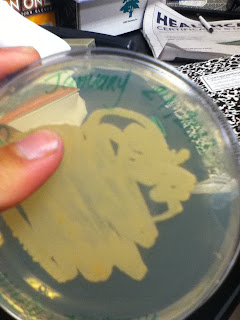
(Pictures of TSB + Rid-X bacteria colony)
Streaking instructions
- 1) Strike once (horizontal/vertical)
- 2) Use a different inoculation loop and streak from the previous streak (about the tip of) to another vertical/horizontal or slanted streak.
- 3) Use a different inoculation loop and repeat step 2.
With new TSA petri dishes and my sample of TSB and Rid-X, I used the method of streaks. (I was also told for future purposes that when labeling petri dishes, it's best to write at the perimeter of the dish for better viewing instead of the text covering the results). I then placed the two petri dishes in the incubator so I can gram stain them next week.
I also had my soil + TSB samples from Tuesday and also applied the new streaking technique I learned. I also placed those in the incubator for next week's gram stain.
First Official Gram Stain
I was eager to learn how to gram stain (seeing as it was the last or close to last step to identify bacteria) and remember how to apply it for future projects. I looked up different ways how to gram stain, but ultimately Josh helped me out and the visual presentation was more helpful than reading off from text. I thought it was going to be very difficult and have many steps (which it did), but it became simple once I got the hang of it.
Procedures
- Gather materials (lighter, candle-like object*, clean slides, China marker, Crystal Violet, Distilled water, Gram Iodide, Decoloizer, Safromin, petri dishes with samples, inoculated loops, clothespin)
- Make a circle on the center of the slide using the China marker for a target.
- Apply about a drop or two of distilled water in the target.
- Next, use an inoculated loop to swab the sample from the petri dish (TSB+Rid-X) and add it to the distilled water.
- I clipped the slide to the clothespin, and used lighter to light fire on the candle-like object*.
- With the fire present, I hovered my slide on top of the fire with a few back and forth movements until the water evaporated.
- I removed the fire, and started the staining process. ***By the way, the method is called Fixing before staining.
Gram Staining
- With slide, I added a Crystal Violet on the target and waited for 30 seconds. (Was done over sink with gloves)
- Next, I washed the slide with Distilled Water for 5 seconds.
- I then added Gram Iodide for 60 seconds.
- I washed the slide with Distilled Water and for 5 seconds.
- I then added Decolorizer for 1-5 seconds.
- I washed the slide with Distilled Water for 1-5 seconds.
- I then added Safromin for about 10-20 seconds.
- Lastly, I washed it off with Distilled Water.
Letting the slide dry off, it became maroon-purple like color and was ready to be seen under the microscope. The results were tricky as I saw a combination of Cocci and Bascilus bacteria. This is why I redid the streaking to get a better view of what bacteria is in the Rid-X. I will be doing this next Tuesday for a new sample + Soil and TSB samples.
*I will find out what the object is called.
*I will find out what the object is called.






